Publications
You can also browse my Google Scholar profile
Journal articles Cover gallery Patents Book chapters
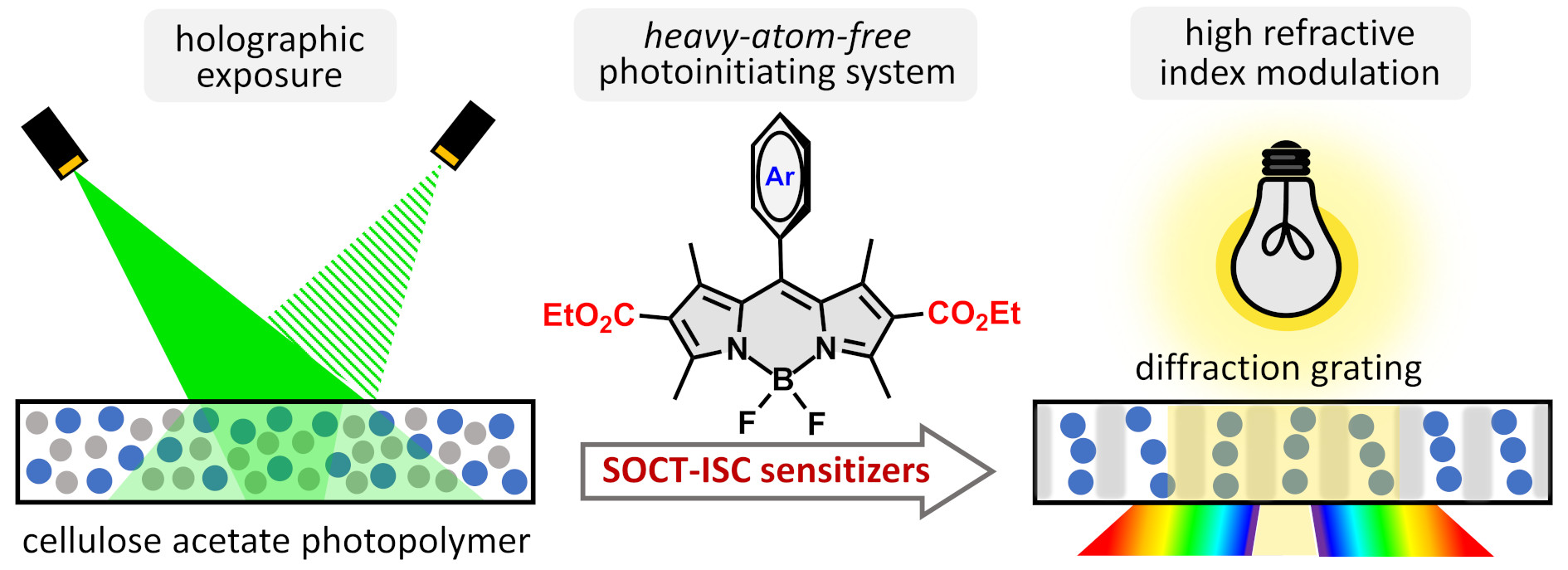
38. Diethoxycarbonyl-BODIPYs as heavy-atom-free photosensitizers for holographic recording in cellulose acetate photopolymer.
A. Sheehan, T. Mikulchyk, C. De Castro, S. Karuthedath, W. Althobaiti, Gul Sabad-e-, H.J. Byrne, M. Dvoracek, F. Laquai, I. Naydenova, M.A. Filatov,* J. Mater. Chem. C, 2023, 11, 15084-15096. LINK (open access)
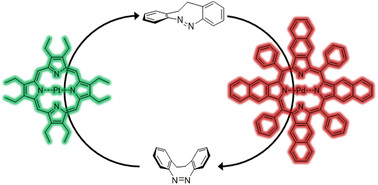
37. Triplet sensitization enables bidirectional isomerization of diazocine with 130 nm redshift in excitation wavelengths.
J. Isokuortti, T. Griebenow, J.-S. von Glasenapp, T. Raeker, M.A. Filatov, T. Laaksonen, R. Herges, N.A. Durandin, Chem. Sci., 2023, 14, 9161-9166. LINK (open access)
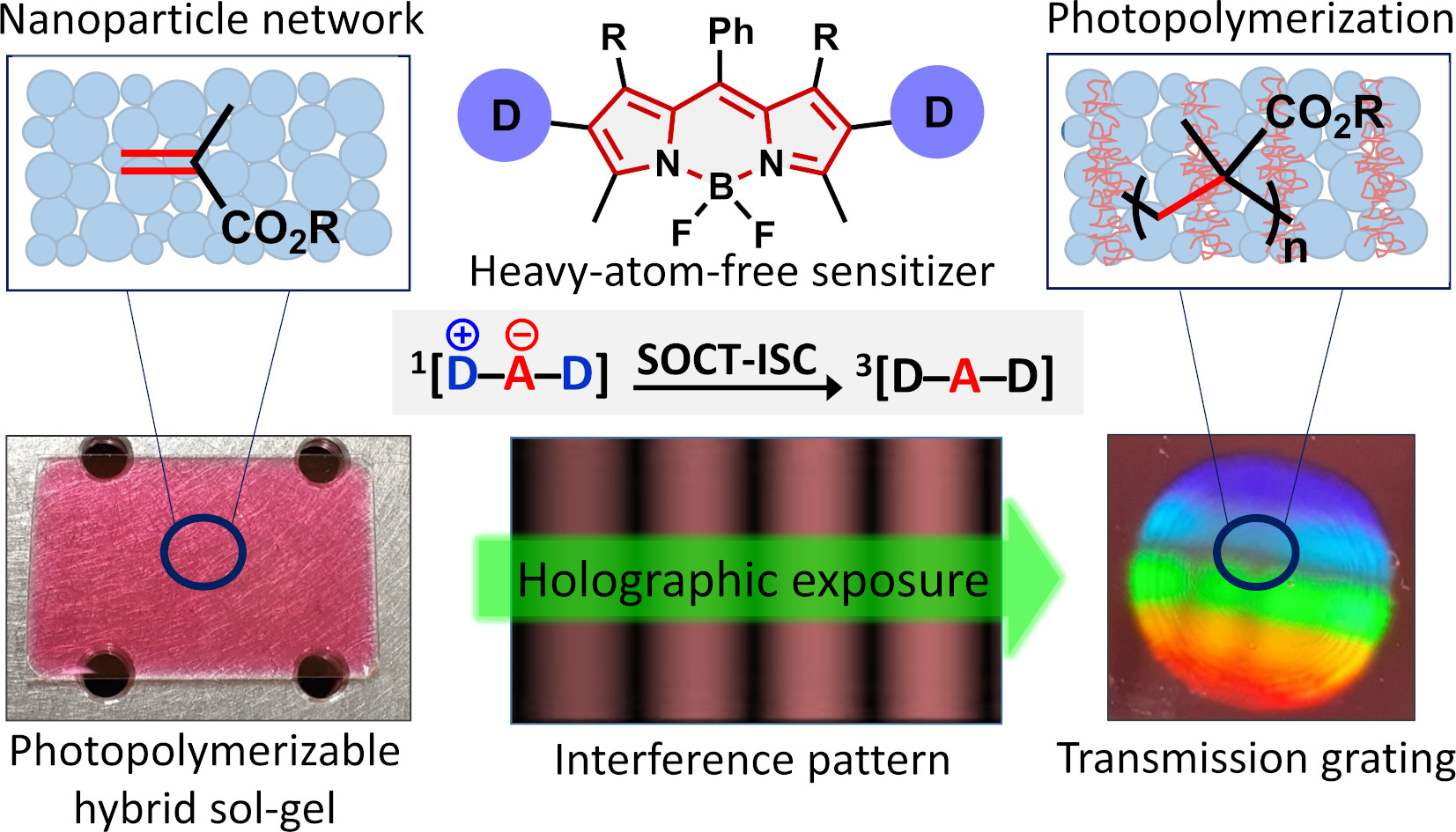
36. Charge Transfer Mediated Triplet Excited State Formation in Donor-Acceptor-Donor BODIPY: Application for Recording of Holographic Structures in Photopolymerizable Glass.
T. Mikulchyk, S. Karuthedath, C. De Castro, A.A. Buglak, A. Sheehan, A. Wieder, F. Laquai, I. Naydenova, M.A. Filatov,* J. Mater. Chem. C., 2022, 10, 11588-11597. LINK (open access)
Highlighted on the back cover.
35. Ruthenium(II) complexes with phosphonate-substituted 1,10-phenanthroline ligands: synthesis, characterization and use in organic photocatalysis.
G.V. Morozkov, A.S. Abel,* M.A. Filatov, S.E. Nefedov, V.A. Roznyatovsky, A.V. Cheprakov, A.Yu. Mitrofanov, I.S. Ziankou, A. Averin, I.P. Beletskaya, J. Michalak, C. Bucher,* L. Bonneviot, A. Bessmertnykh-Lemeune,* Dalton Trans., 2022, 51, 13612-13630. LINK
Highlighted on the front cover. Dalton Transactions HOT Articles Collection.

34. BODIPY–pyrene donor–acceptor sensitizers for triplet–triplet annihilation upconversion: the impact of the BODIPY-core on upconversion efficiency.
N. Kiseleva, M.A. Filatov, J.C. Fischer, M. Kaiser, M. Jakoby, D. Busko, I.A. Howard, B.S. Richards, A. Turshatov*, Phys. Chem. Chem. Phys., 2022, 24, 3568-3578. LINK (open access)
2022 PCCP HOT Articles Collection.

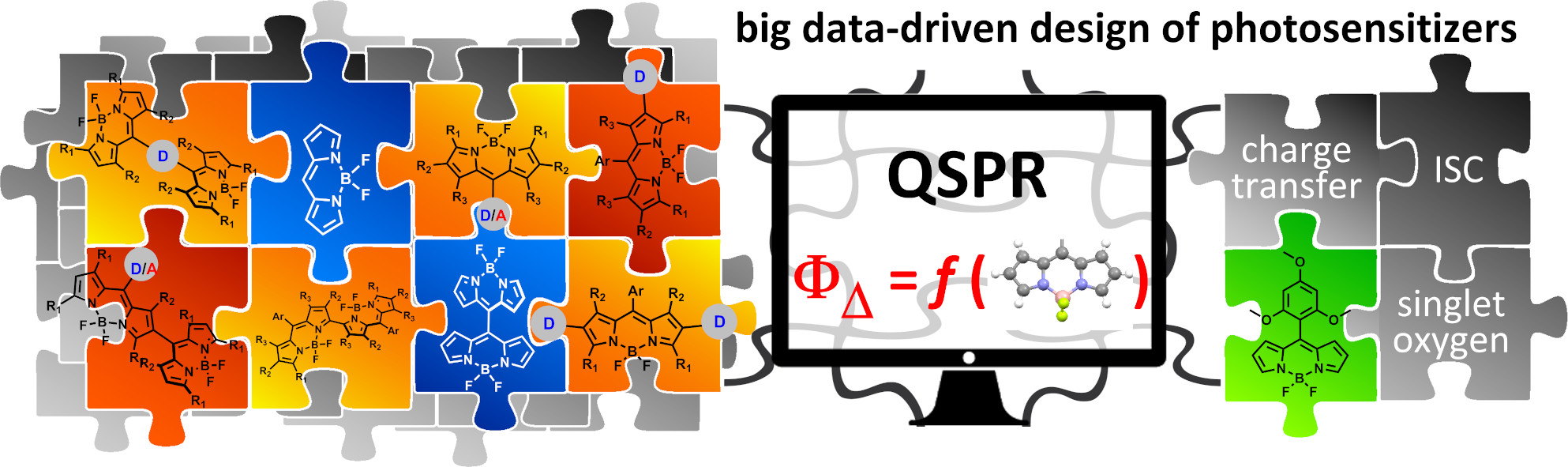
33. Quantitative Structure–Property Relationship Modelling for the Prediction of Singlet Oxygen Generation by Heavy‐atom‐free BODIPY Photosensitizers.
A.A. Buglak, A. Charisiadis, A. Sheehan, C.J. Kingsbury, M.O. Senge*, M.A. Filatov* Chem. Eur. J., 2021, 27, 9934-9947. LINK (open access)
32. Expanding Azobenzene Photoswitching into Near-Infrared via Endothermic Triplet Energy Transfer.
J. Isokuortti, K. Kuntze, M. Virkki, Z. Ahmed, E. Vuorimaa-Laukkanen, M.A. Filatov, A. Turshatov, T. Laaksonen, A. Priimagi, N. Durandin, Chem. Sci., 2021, 2021,12, 7504-7509. LINK (open access)

31. Determination of Upconversion Quantum Yields Using Charge-Transfer State Fluorescence of Heavy-Atom-Free Sensitizer as a Self-Reference.
N. Kiseleva, D. Busko, B.S. Richards, M.A. Filatov*, A. Turshatov* J. Phys. Chem. Lett., 2020, 11, 6560-6566. LINK

30. Singlet Oxygen Generation by Porphyrins and Metalloporphyrins Revisited: a Quantitative Structure-Property Relationship (QSPR) Study.
A.A. Buglak,* M.A. Filatov, M.A. Hussain, M. Sugimoto J. Photochem. Photobiol. A, 2020, 43, 112833. LINK
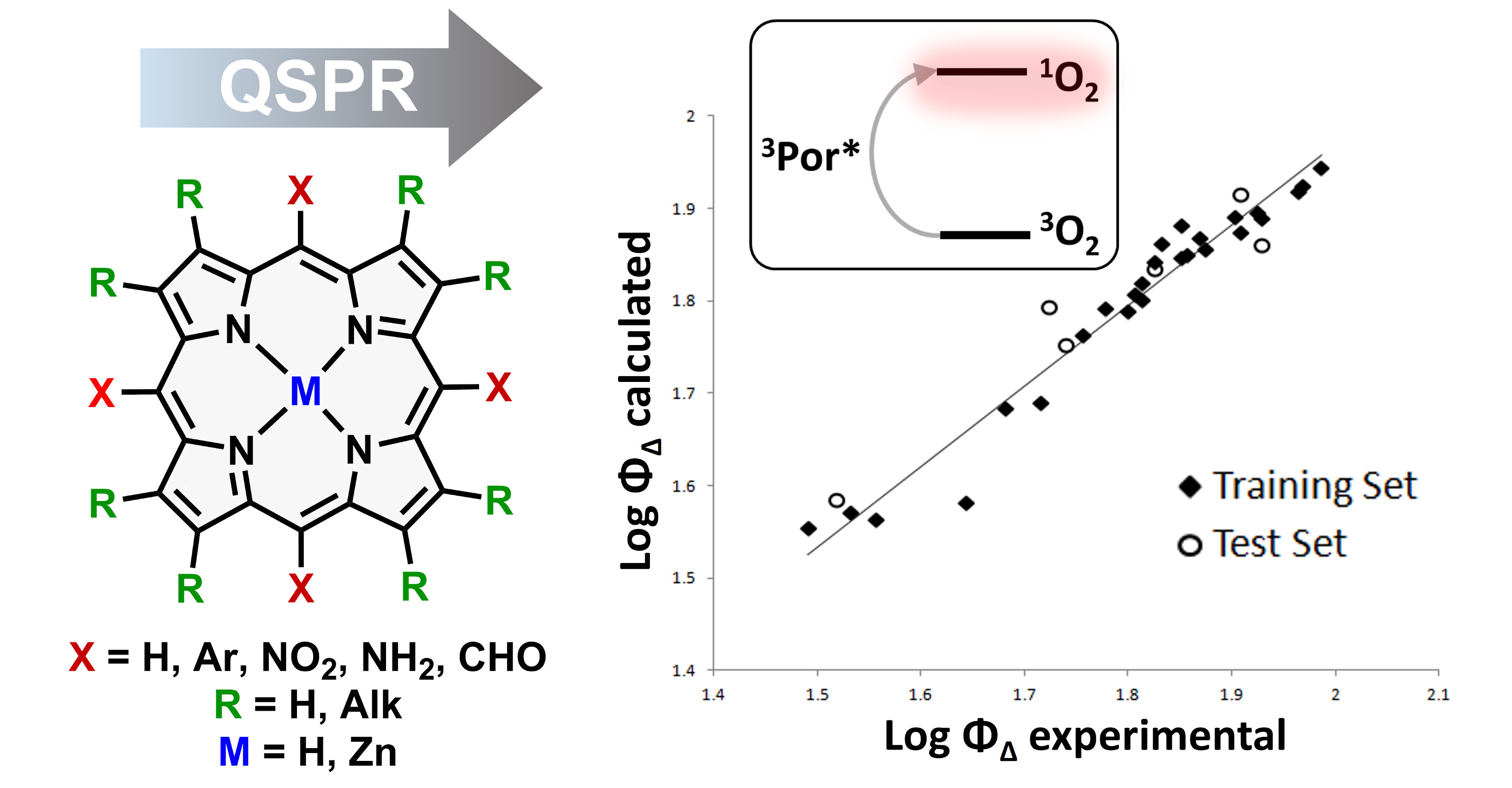
29. Heavy-atom-free BODIPY Photosensitizers with Intersystem Crossing Mediated by Intramolecular Photoinduced Electron Transfer.
M.A. Filatov* Org. Biomol. Chem., 2020, 18, 10-27. LINK (open access)

28. In vitro cytotoxicity of a library of BODIPY-anthracene and -pyrene dyads for application in photodynamic therapy.
S. Callaghan, M.A. Filatov, H. Savoie, R.W. Boyle,* M.O. Senge* Photochem. Photobiol. Sci., 2019, 18, 495-504. LINK
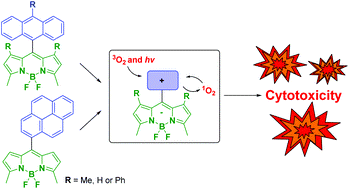
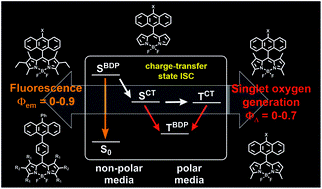
27. BODIPY‐Pyrene and Perylene Dyads as Heavy‐Atom‐Free Singlet Oxygen Sensitizers.
M.A. Filatov*, S. Karuthedath, P.M. Polestshuk, S. Callaghan, K. Flanagan, T. Wiesner, F. Laquai, M.O. Senge* ChemPhotoChem, 2018, 2, 606-615. LINK
Top downloaded paper 2018-2019.
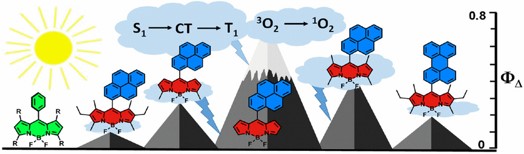
26. Control of triplet state generation in heavy atom-free BODIPY–anthracene dyads by media polarity and structural factors.
M.A. Filatov*, S. Karuthedath, P.M. Polestshuk, S. Callaghan, K. Flanagan, M. Telitchko, T. Wiesner, F. Laquai, M.O. Senge* Phys. Chem. Chem. Phys., 2018, 20, 8016-8031. LINK
PCCP 2018 Hot Articles Collection.
25. The Janus-Faced Chromophore: A Donor-Acceptor Dyad with Dual Performance in Photon Up-conversion.
N. Kiseleva, M.A. Filatov*, M. Oldenburg, D. Busko, M. Jakoby, I.A. Howard, B.S. Richards, M.O. Senge, S.M. Borisov, A. Turshatov* Chem. Commun., 2018, 54, 1607-1610. LINK

24. Generation of Triplet Excited States via Photoinduced Electron Transfer in meso-anthra-BODIPY: Fluorogenic Response toward Singlet Oxygen in Solution and in Vitro.
M.A. Filatov*, S. Karuthedath, P.M. Polestshuk, H.Savoie, K.J. Flanagan, C. Sy, E. Sitte, M. Telitchko, F. Laquai, R.W. Boyle, M.O. Senge* J. Am. Chem. Soc., 2017, 139, 6282−6285. LINK (open access)
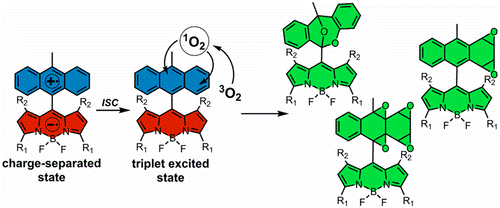
23. Delayed release singlet oxygen sensitizers based on pyridone-appended porphyrins.
S. Callaghan, M.A. Filatov*, E. Sitte, H. Savoie, R.W. Boyle, K.J. Flanagan, and M.O. Senge* Photochem. Photobiol. Sci., 2017, 16, 1371-1374. LINK
Highlighted on the front cover.

22. Molecular devices based on reversible singlet oxygen binding in optical and photomedical applications.
M.A. Filatov* and M.O. Senge Mol. Syst. Des. Eng., 2016, 1, 258-272. LINK
Highlighted on the front cover. Top 10 most read articles.
21. Protection of Densely Populated Excited Triplet State Ensembles Against Deactivation by Molecular Oxygen.
M.A. Filatov*, S. Baluschev,* K. Landfester* Chem. Soc. Rev., 2016, 45, 4668-4689. LINK (open access)
Highlighted on the front cover.

20. Studying the intersystem crossing rate and triplet quantum yield of meso-substituted porphyrins by means of pulse train fluorescence technique.
T.G.B. de Souza, M.G. Vivas, C.R. Mendonça, S. Plunkett, M.A. Filatov, M.O. Senge, L. De Boni* J. Porphyrins Phthalocyanines, 2016, 20, 1–10. LINK
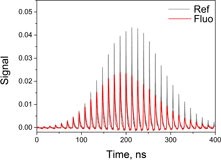
19. Interplay between singlet and triplet excited states in a conformationally locked donor–acceptor dyad.
M.A. Filatov*, F. Etzold, D. Gehrig, F. Laquai, D. Busko, K. Landfester, S. Baluschev Dalton Trans., 2015, 44, 19207-19217. LINK (open access)
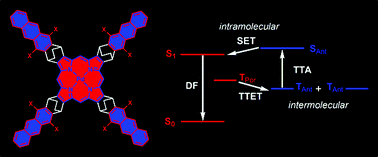
18. meso-Tetraphenylporphyrin with a pi-system extended by fusion with anthraquinone.
M.A. Filatov*, E. Heinrich, K. Landfester, S. Baluschev Org. Biomol. Chem., 2015, 13, 6977-6983. LINK (open access)
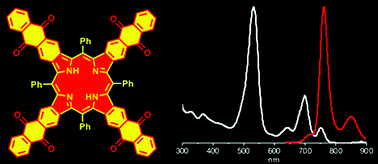
17. Reversible Oxygen Addition on a Triplet Sensitizer Molecule: Protection from Excited States Depopulation.
M.A. Filatov*, E. Heinrich, D. Busko, I.Z. Ilieva, K. Landfester, S. Baluschev Phys. Chem. Chem. Phys., 2015, 17, 6501-6510. LINK (open access)
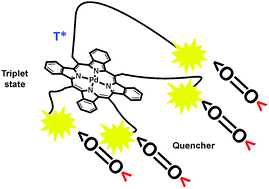
16. Extending the infrared limit of oxygenic photosynthesis.
M.A. Filatov, S. Ritz, I. Ilieva, V. Mailander, K. Landfester, S. Baluschev* SPIE Newsroom, 2014, doi: 10.1117/2.1201403.005378. LINK
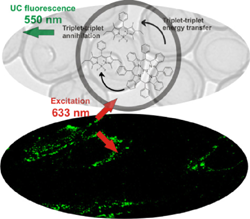
15. Fabrication of low-power upconverting nanocapsules for bioimaging in red and far-red spectral regions.
C. Wohnhaas, V. Mailänder, M. Dröge, M.A. Filatov, D. Busko, Y. Avlasevich, Stanislav Baluschev,* T. Miteva, K. Landfester, A. Turshatov* Macromolecular Bioscience, 2013, 13, 1422−1430. LINK
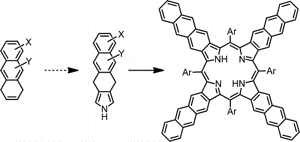
14. Tetraanthraporphyrins: synthesis, structure and optical properties.
M.A. Filatov*, S. Baluschev, I.Z. Ilieva, V. Enkelmann, T. Miteva, K. Landfester, S. Aleshchenkov, A.V. Cheprakov* J. Org. Chem., 2012, 77, 11119−11131. LINK
13. Decoupling the Artificial Special Pair to Slow Down the Rate of Singlet Energy Transfer.
P.D. Harvey,* A. Langlois, M.A. Filatov, D. Fortin, K. Ohkubo, S. Fukuzumi,* R. Guilard* J. Porphyrins Phthalocyanines, 2012, 16, 8-10. LINK

12. The Synthesis of Highly Basic π-Extended Porphyrins by Palladium Catalyzed Amination.
E.R. Ranyuk, M.A. Filatov, A.D. Averin, A.V. Cheprakov,* I.P. Beletskaya Synthesis, 2012, 3, 393-398. LINK
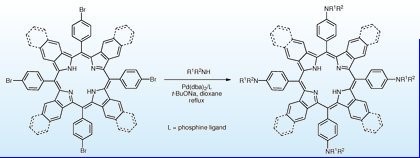
11. Near infrared dipyrrin-based fluorogenic chelators for metal ions.
S. Thyagarajan, B. Ghosh, M.A. Filatov, A.V. Moore, A.V. Cheprakov, S.A. Vinogradov* Proc. SPIE, 2011, 7910, 79100Z. LINK
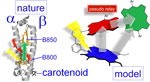
10. Bis- and Trisporphyrin Bio-Inspired Models for Bacterial Antennas and Photosystems.
P.D. Harvey,* M.A. Filatov, R. Guilard* J. Porphyrins Phthalocyanines, 2011, 15, 1-22. LINK
9. The Synthesis of New Tetrabenzo- and Tetranaphthoporphyrins via the Addition Rreactions of 4,7-Dihydroisoindole.
M.A. Filatov and A.V. Cheprakov* Tetrahedron, 2011, 3559-3566. LINK

8. Strong Donor–Acceptor Couplings in a Special Pair-Antenna Model.
M.A. Filatov, F. Laquai, D. Fortin, R. Guilard,* P.D. Harvey* Chem. Comm., 2010, 46, 9176-9178. LINK

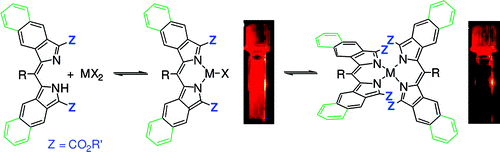
7. π-Extended Dipyrrins Capable of Highly Fluorogenic Complexation with Metal Ions.
M.A. Filatov, A. Y. Lebedev, S.N. Mukhin, S. A. Vinogradov,* A. V. Cheprakov* J. Am. Chem. Soc., 2010, 132, 9552-9554. LINK
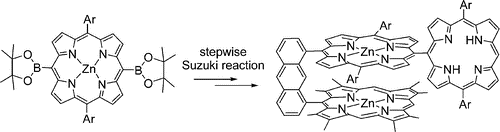
6. Selective Stepwise Suzuki Cross-coupling Reaction for the Modelling of Photosynthetic Donor−Acceptor Systems.
M.A. Filatov, R. Guilard,* P.Harvey* Org. Lett., 2010, 12, 196-199. LINK
5. The Dihydroisoindole Approach to π-Extended Porphyrins.
A.V. Cheprakov* and M.A. Filatov J. Porphyrins and Phthalocyanines, 2009, 13, 291-303. LINK

4. Effects of Structural Deformations on Optical Properties of Tetrabenzoporphyrins: Free-bases and Pd Complexes.
A.Y. Lebedev, M.A. Filatov, A.V. Cheprakov,* S.A. Vinogradov* J. Phys. Chem. A., 2008, 112, 7723-7733. LINK
3. Synthesis of 5,15-Diaryltetrabenzoporphyrins.
M.A. Filatov, A.Y. Lebedev, S.A. Vinogradov,* A.V. Cheprakov* J. Org. Chem., 2008, 73, 4175-4185. LINK

2. A Facile and Reliable Method for the Synthesis of Tetrabenzoporphyrins from 4,7-Dihydroisoindole.
M.A. Filatov, A.V. Cheprakov,* I.P. Beletskaya Eur. J. Org. Chem., 2007, 3468-3475. LINK

1. Novel Synthesis of Substituted Tetraaryltetrabenzoporphyrins.
O.S. Finikova, A.V. Cheprakov, S.Y. Chernov, M.A. Filatov, S.A. Vinogradov, I.P. Beletskaya* Doklady Chemistry, 2003, 391, 222-224. LINK
