Photoinduced electron transfer and SOCT-ISC
Photoinduced electron transfer in the excited state is a common phenomenon, observed for dye molecules containing electron-donor and acceptor groups. Electronic structure of such molecules provides a possibility that after light absorption the electron is transferred from the donor unit to the acceptor, which creates a highly dipolar excited state - charge-transfer (CT) state.
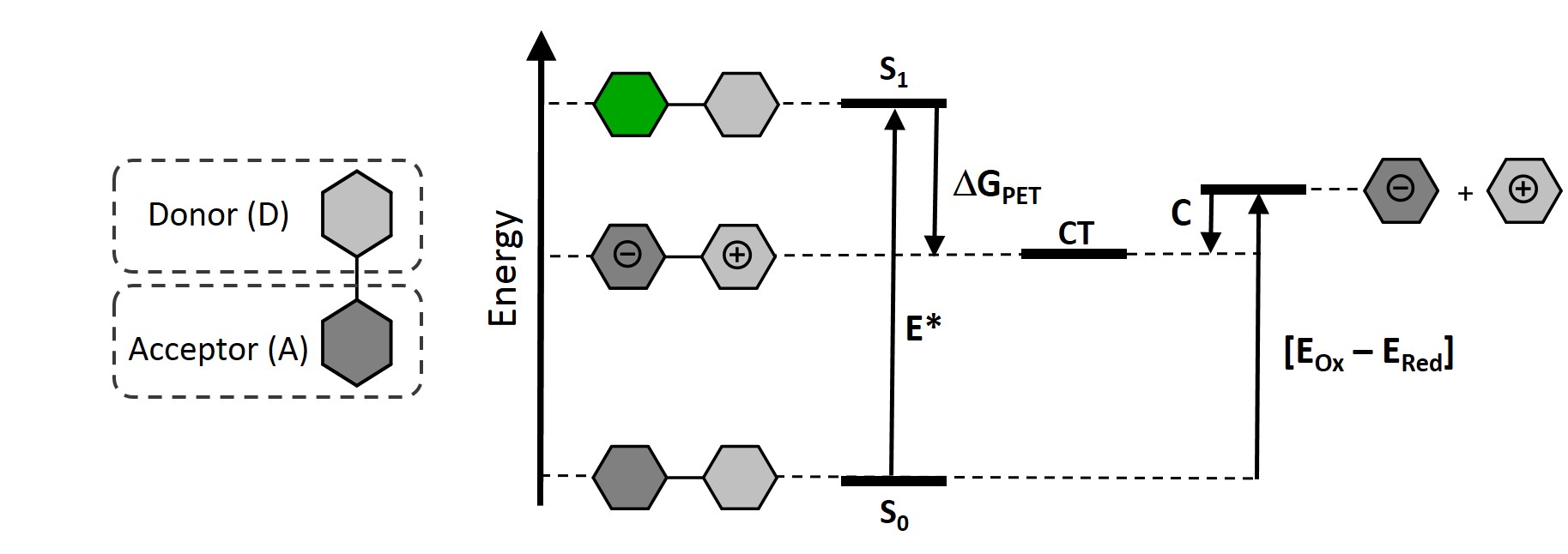 Photoinduced electron transfer in a donor-acceptor dyad, resulting in CT state formation. |
Recombination of CT states (CR) can result in the formation of a local triplet excited state with high yields via the so-called spin-orbit charge-transfer (SOCT-ISC) mechanism:
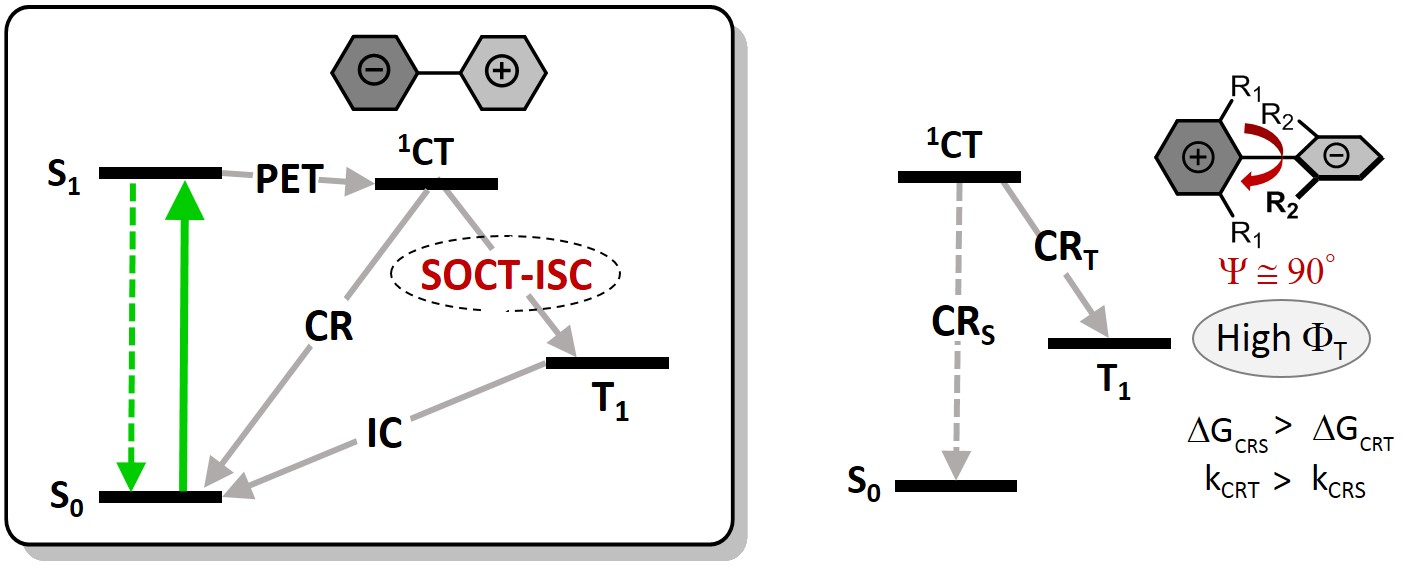 Triplet excited states formation from CT states via SOCT-ISC. |
Notably, SOCT-ISC mechanism does not require introduction of transition metals or other heavy atoms into the molecule. Triplet sensitizers operating via SOCT-ISC are highly advantageous due to the following reasons: 1) they are cheaper and easier to prepare compared common sensitizers, such as transition metal complexes and halogenated dyes; 2) they are non-toxic and environmentally friendly; 3) they possess intense absorption the visible region (400-600 nm). Application of these molecules in photocatalysis, triplet-triplet annihilation upconversion (TTA-UC) and photodynamic therapy (PDT) is currently a subject of active investigations.
Along with collaborators we studying and optimizing the performance of donor acceptor systesm, in particular the ISC rates and triplet state yields, sensitivity of charge transfer to environmental parameters (polarity, viscosity, magnetic field), absorption in spectral region of interest (500-800 nm). Our goal is to extend the usefulness of SOCT-ISC phenomenon in various applications.
