Synthetic chemistry
Porphyrins with extended π-systems
The fusion of aromatic rings to porphyrin core has a strong influence on electronic properties, particularly reducing HOMO−LUMO gap and shifting the absorption and emission into near-infrared (NIR) region. Because of these properties, such molecules are attractive for numerous applications, such as NIR photodetectors, photovoltaics, semiconductors, and two-photon absorbing materials. Unlike other porphyrins with extended conjugated π-system, they possess very sharp absorption bands and an optical window between Soret and Q-bands.
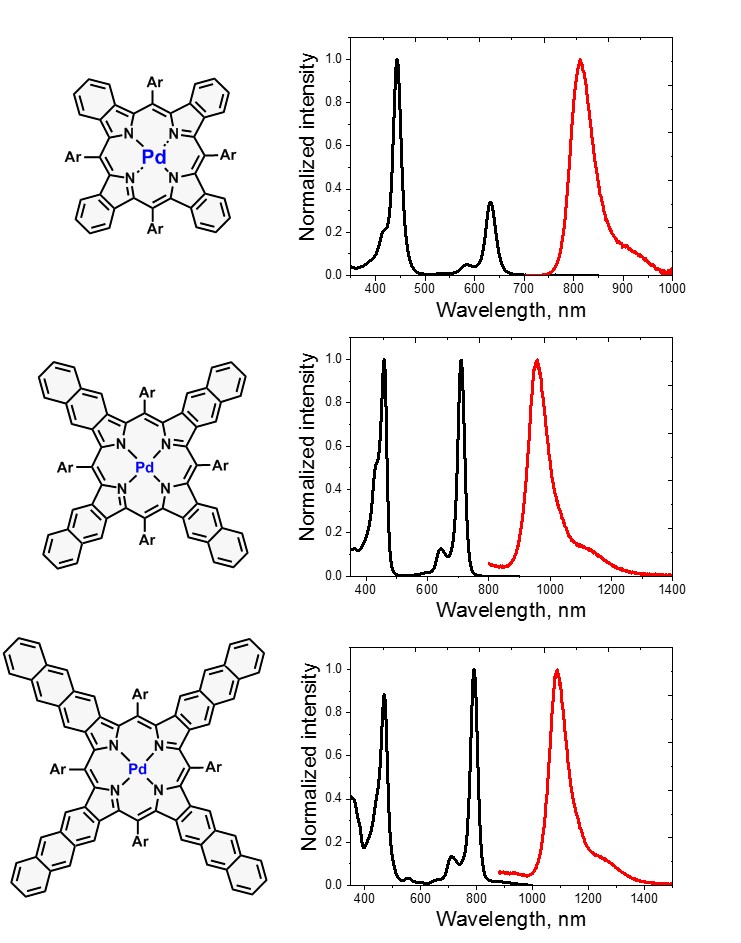 Absorption and phosphorescence spectra of palladium(II) tetrabenzo-, tetranaphtho- and tetraanthraporphyrins. | 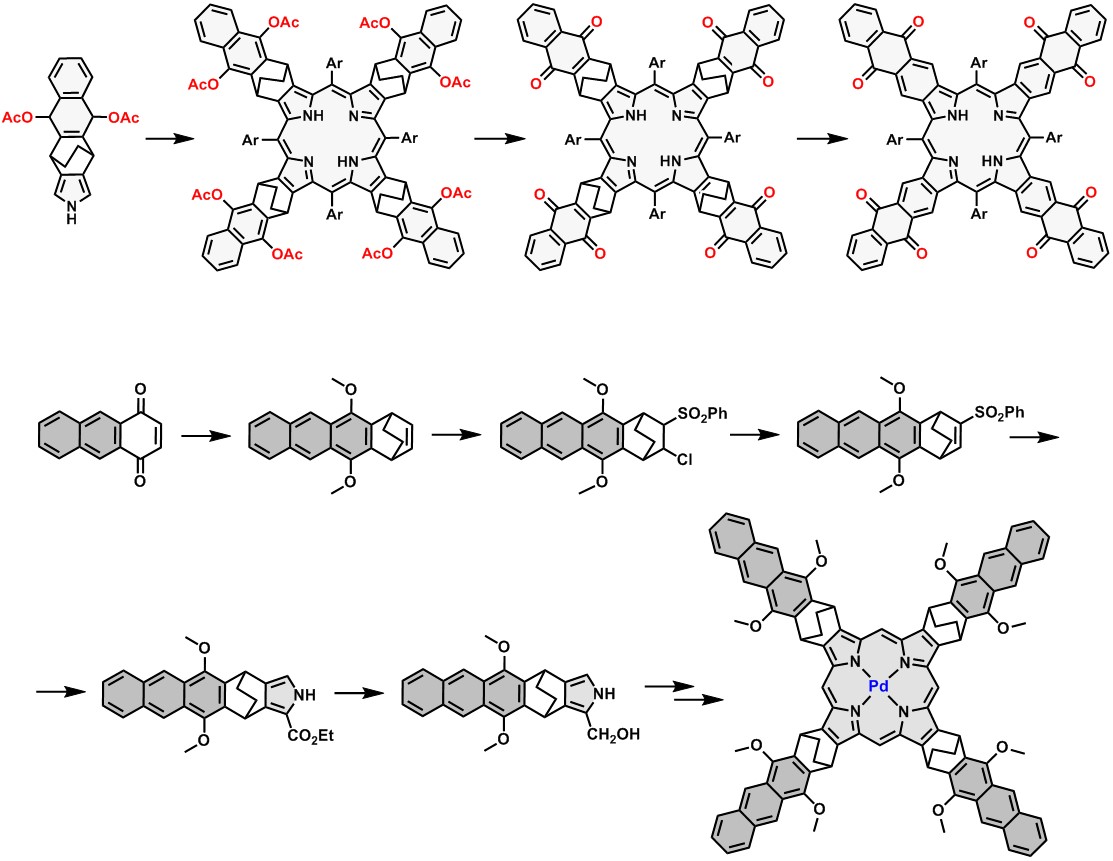 Example synthetic approaches to porphyrins with π-extended system and corresponding conformationally rigid dyads. |
Despite promising properties, these molecules are still scarcely investigated because available synthetic methods have been very limited until recently. My work in this area is focused on synthesis and spectroscopic studies of free-base and metal complexes of novel pi-extended porphyrins, e.g. tetrabenzo-, tetranaphtho-, tetraanthraporphyrins and corresponding conformationally rigid systems.
I am particularly interested in application of these molecules in TTA-UC. In combination with appropriate emitters they enable upconversion under excitation with IR-part of the sun radiation (λ > 700 nm).
BODIPY dyes
Boron-dipyrromethene (BODIPY) is named in the literature as the “little sister” of porphyrins. BODIPY represent one of the most promising classes of dyes due to the ease of synthesis and derivatization, alongside tunability of their photophysical properties. A remarkable feature of BODIPY dyes is the ease of tuning their electronic properties via chemical modification. The excited state transitions in BODIPY chromophore can be finely tuned by the introduction of substituents into the pyrrolic positions. As is shown in figure below the parent BODIPY possesses very strong fluorescence in solution due to high radiative decay rates. Alternatively, corresponding heavy atom-containing BODIPYs show very strong spin-orbit coupling and high triplet state yields, which accounts for its uses as photosensitizers.
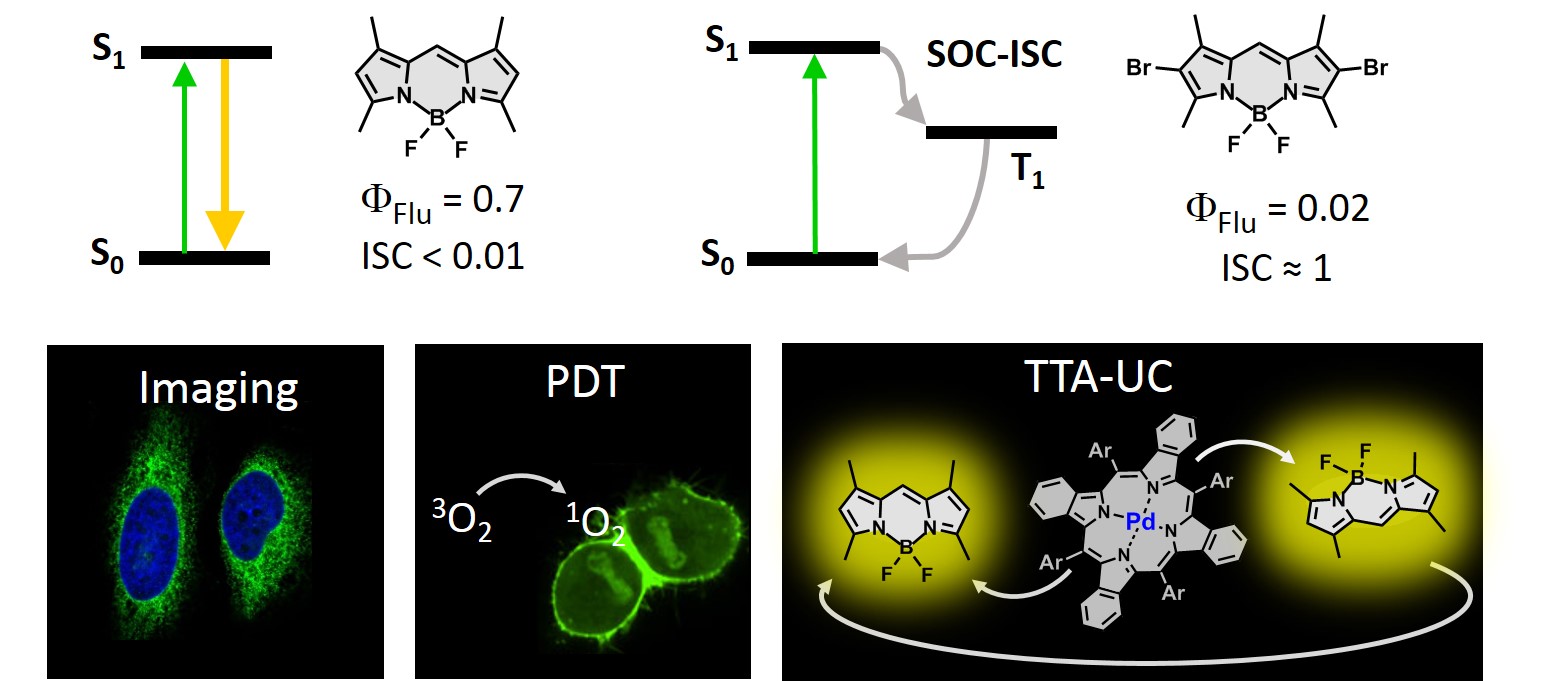 The effect of substitution on the excited state transitions in BODIPY chromophores. Applications of BODIPY dyes - bioimaging, PDT, photon upconversion. |
My research in the area of BODIPYs is focused on the development of new dyes which we tentatively call Janus-faced chromophores. These molecules are capable of either strong fluorescence or triplet excited state generation, depending on parameters of the environment, applied external stimuli or activating species. The application of these novel compounds and corresponding materials is expected to significantly increase the performance and functionality of target applications whilst reducing synthetic efforts and technology costs with respect to conventional methods, which ultimately will allow for their use in industry. An example of such dyes, BODIPY-anthracene dyad (BAD) shown below behaves as a typical fluorophore in non-polar environment, exhibiting high fluorescence quantum yield (up to 0.9) and negligible ISC. Alternatively, in polar solvent BAD efficiently generates triplet excited states.
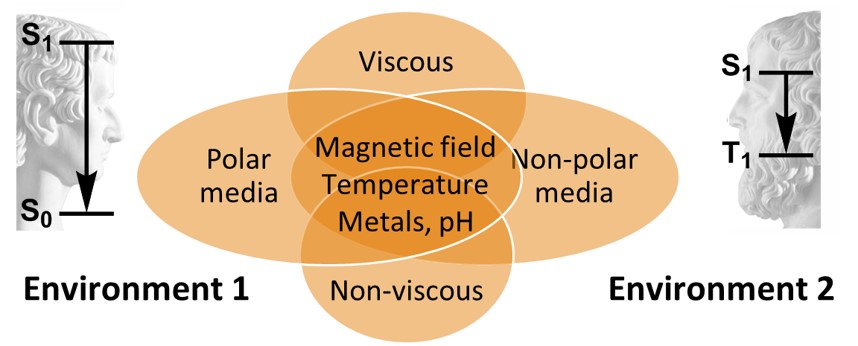 A concept of Janus-faced chromophores. | 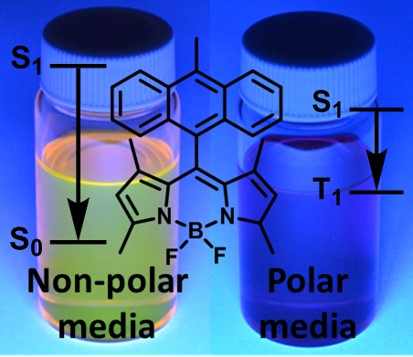 BAD in polar and non-polar solvents. |
Water-soluble derivatives of such molecules induce a significant cytotoxic effect on the cells and can be applied as photosensitizers in photodynamic therapy. They can be obtained, e.g. by substitution of fluorine atoms in the BODIPY and subsequent introduction of sulfobetaine groups:
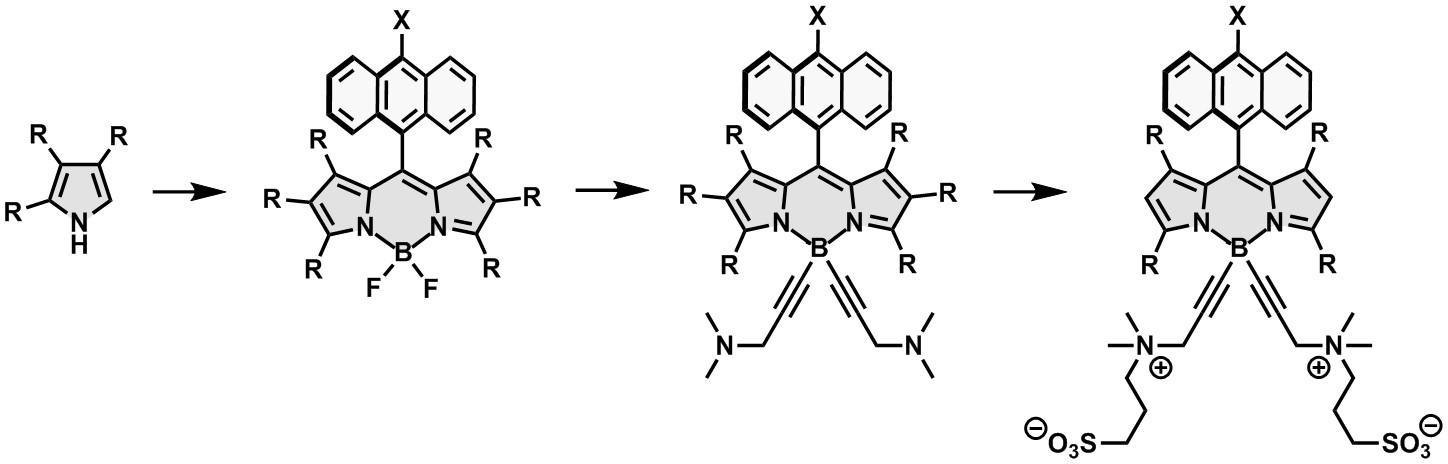 Synthetic approach to water-soluble BODIPY sensitizers for PDT. |
